Explain How Electronegativity Difference Makes a Bond Polar or Nonpolar
Cl has an EN electronegativity of 35 but C on the other end is 25 and H is 21 so there is a difference and that produces a polar molecule. In a polar covalent bond the electrons are not equally.

Dublin Schools Lesson Electronegativity
There is a charge bui View the full answer.
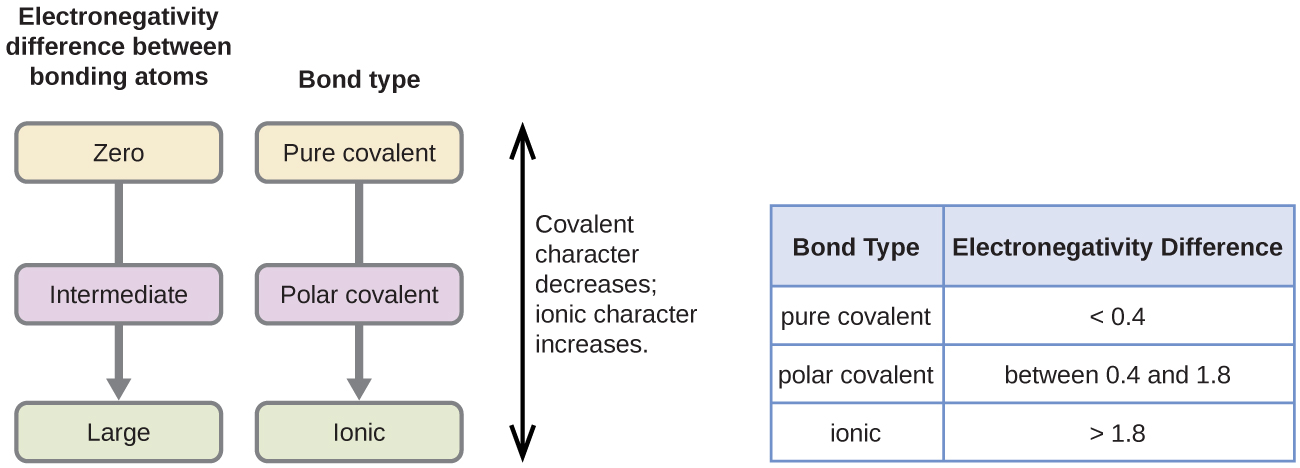
. If the difference in electronegativity is less than 04 the bond is essentially nonpolar If there are no polar bonds the molecule is nonpolar. In non-polar covalent bonds electrons are equally shared by the two atoms participating in making the bond. The rule is that when the electronegativity difference is greater than 20 the bond is considered ionic.
The atoms electronegativity difference is greater than 04. Explain the difference between a nonpolar covalent bond a polar covalent bond and an ionic bond. The greater difference in electronegativity between the bonded atoms the greater is the polarity.
Electronegativity is the tendency of an atom to attract a shared pair of electrons toward itself. Therefore they are electrically charged. A covalent bond is formed through the transfer of electrons from one atom to another.
The Lewis structure determines whether an entire molecule is polar or essentially nonpolar. The electron cloud in these molecules are distorted. Polar covalent bonds share electrons equally while nonpolar covalent bonds share electrons unequally.
If we think of this molecule as a chain the CH3 is on one end Cl is on the other with CH2 between. Similarly what makes a polar covalent bond. Polar covalent bonding is a type of chemical bond where a pair of electrons is unequally shared between two atoms.
The main difference between polar and nonpolar molecules is. In a nonpolar covalent bond the atoms share the electron equally. If the electronegativity difference usually called ΔEN is less than 05 then the bond is nonpolar covalent.
If the ΔEN is between 16 and 20 and if a metal is involved then the bond is considered ionic. So lets review the rules. If only nonmetals are involved the bond is considered polar covalent.
Polar molecules occur when there is an electronegativity difference between the bonded atoms. A polar covalent bond is when two atoms are not sharing an electron equally. Polar solvents are the solvents that have a polar bond the basis of which is the electronegativity difference between constituent atoms.
These molecules have positive and negative charges on the opposite ends. Covalent bonds which are non-polar are made by two atoms with similar electronegativities. If the electronegativity difference usually called ΔEN is less than 05 then the bond is nonpolar covalent.
Which of the following statements is TRUE. If the electronegativity difference between the atoms is. Difference between polar and non - polar co valent bond.
Since there is fully equal sharing there is no dipole moment in these molecules. It is not possible for two atoms to share more than two electrons. Nonpolar bonds have an electronegativity difference of less than 04 and have an equal sharing of the electron pair.
Single bonds are shorter than double bonds. If the ΔEN is between 05 and 16 the bond is considered polar covalent 3. There isnt that much difference so it may not be highly polar but thats the principle behind it.
Polar bonds have high melting point surface tension boiling point and low vapour pressure. Non-polar covalent bonds have no defined axis or axes compared to polar covalent bonds. Polar covalent bond.
A polar covalent bond is formed when the electronegativity difference is between 5 and 17. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. The rule is that when the electronegativity level is greater than 2 the bond is considered ionic.
When two atoms with 04 electronegativity difference are bonded polar molecules are formed. Polar molecules interact with other polar substances. Studies show that nonpolar moleculesnonpolar covalent bonds are symmetric.
Polar covalent Bond formed between the element which have different electronegativity. Meanwhile polar molecules are asymmetric because they contain lone pairs of. Polar covalent bonds are made by two atoms with different electronegativities but the different should not be exceeding 17.

4 8 Polar Covalent Bonds And Electronegativity Chemistry Libretexts

6 4 Polarity Of Molecules Introductory Chemistry

Videoquiz Definition Electronegativity Polar Dipole Electron Affinity
No comments for "Explain How Electronegativity Difference Makes a Bond Polar or Nonpolar"
Post a Comment